
Atomic Structure and the Periodic Table
Every substance is made up of atoms. An atom is the smallest part of an element that can exist. Atoms have a central nucleus, made up of positively charged protons and neutral charged neutrons. It is surrounded by negatively charged electrons, arranged into shells.
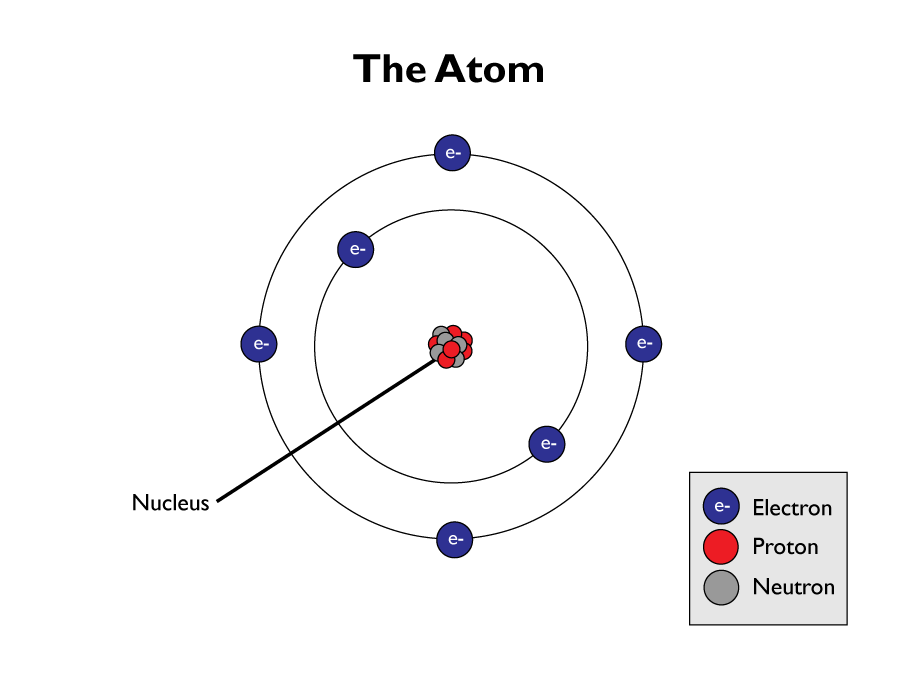
The overall mass of an atom is concentrated in the nucleus. The nucleus is also tiny in comparison to the overall size of an atom. Electron shells cover much of an atoms space. The volume of their orbits dictates the overall size of an atom. Their mass is extremely small compared to protons and neutrons.
The nucleus is minute compared to the size of the atom as a whole. The radius of an atom is 1 x 10-10 m. The radius of a nucleus is 1 x 10-14 m.
The nucleus has an overall positive charge because it’s protons.
Every atom has no overall charge, this is because they contain an equal amount of positively charged protons, and negatively charged electrons, which cancels each other out.
| Particle | Relative Mass | Relative Charge |
|---|---|---|
| Proton | 1 | +1 |
| Neutron | 1 | 0 |
| Electron | Very small | -1 |
An ion is different to an ordinary atom because it has a different number of protons, to the number of electrons. It therefore has an overall charge. An ion with a 3- charge has three more electrons than protons, it therefore has a negative charge.
Each atom has a designated atomic number which tells you how many protons it has. They also have a mass number that tells you the total number of protons and neutrons.
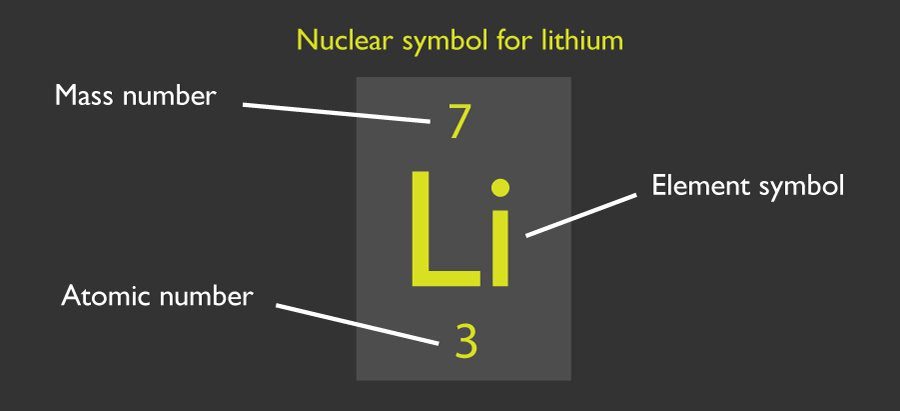
The number of neutrons is given by subtracting the atomic number from the mass number. The number of electrons is equal to the atomic number.
To summarise:
- number of protons = atomic number
- number of electrons = atomic number
- number of neutrons = mass number - atomic number
Elements
There are about 100 different elements. Elements are shown in the periodic table. Each element consists of atoms which all have the same number of protons in their nucleus (they all have the same atomic number).
Isotopes
An isotope of an atom has a different number of neutrons, but the same number of protons and electrons.
To summarise isotopes have:
- The same atomic number (the number of protons)
- different mass number (the number of protons and neutrons)
An isotope is named after the element and the mass number of its atoms. For example carbon-12 is an isotope of carbon with a mass number of 12.
The isotopes of hydrogen are as follows:
| Isotope | Symbol | Protons | Electrons | Neutrons |
|---|---|---|---|---|
| Hydrogen-1 | 1H1 | 1 | 1 | 1 – 1= 0 |
| Hydrogen-2 | 2H1 | 1 | 1 | 2 – 1 = 1 |
| Hydrogen-3 | 3H1 | 1 | 1 | 3 – 1 = 2 |
Each of the hydrogen isotopes have identical chemical properties, since those are determined by the number of electrons and the electron configurations are the same in all three isotopes.
Relative Atomic Mass
The relative atomic mass of an element is the average mass of its atoms, compared to 1/12th the mass of a carbon-12 atom. The relative atomic mass, Ar, of an element is calculated from:
- the mass numbers of its isotopes
- the abundance of these isotopes
You can use the following formula to work out the relative atomic mass (Ar) of an isotope.

For example: The naturally occurring isotopes of copper are Cu-63 and Cu-65. The abundances are 69.2% and 30.8% respectively.
Therefore the relative atomic mass for the isotopes are:
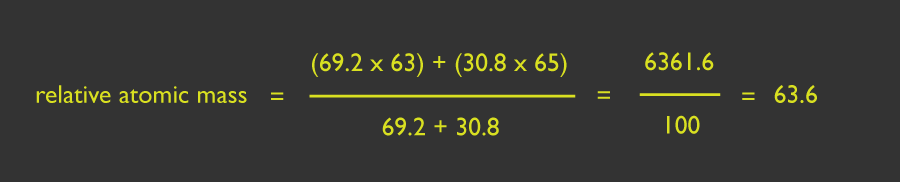
Compounds
Compounds are formed from elements undergoing chemical reactions. Chemical reactions always involve the formation of one or more new substances, and often involve a detectable energy change, usually as a change in temperature.
The atoms that make up compounds, are held together by chemical bonds. It is often difficult to separate these bonds once they have formed, to do this, another chemical reaction is required.
When a bond is made, the nucleus of each atom is completely unaffected; it is the electrons that are either taken, given away, or shared between the atoms.
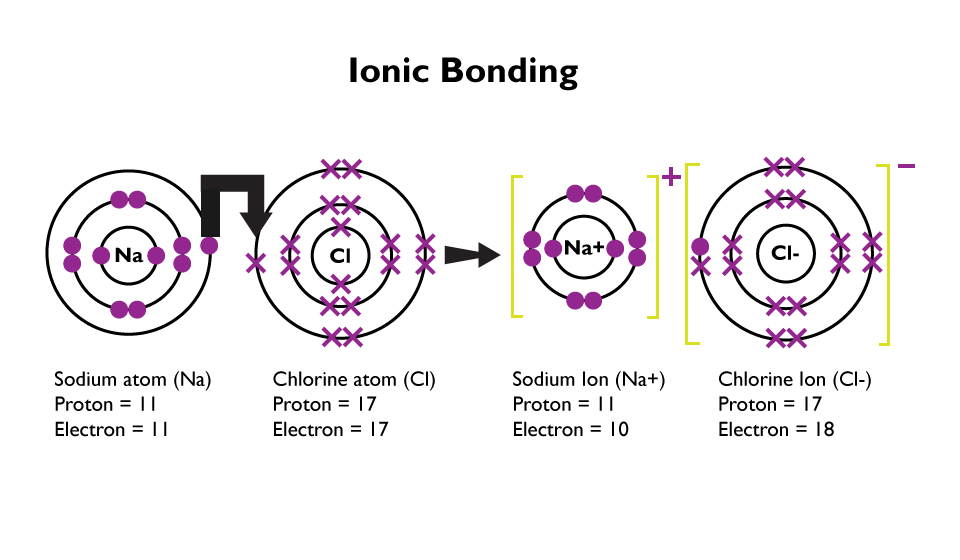
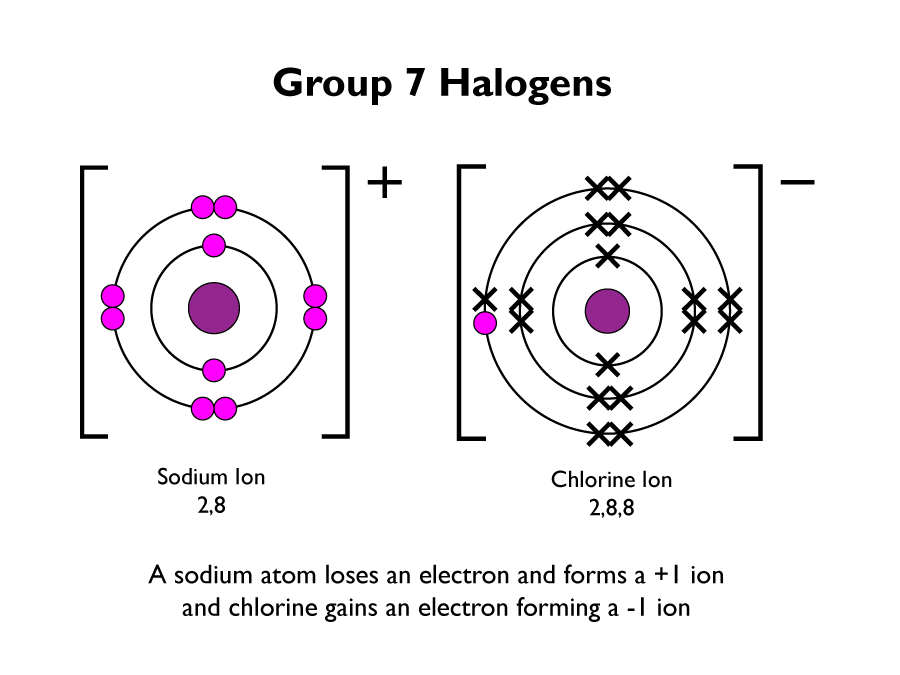
When a metal and a non-metal react, ion’s are produced. Negative ions are produced when a non-metal gains electrons. A positive ion is produced when the metal loses an electron. Ionic bonding occurs when the two oppositely charged ions are attracted to each other. This forms a strong bond between them.
The following diagram is of a sodium atom giving an electron to a chlorine atom.
The compound formed from non-metals reacting consists of molecules. A covalent bond is formed when each atom shares an electron. The following diagram shows a hydrogen chloride atom, bonding covalently, by each atom sharing an electron.
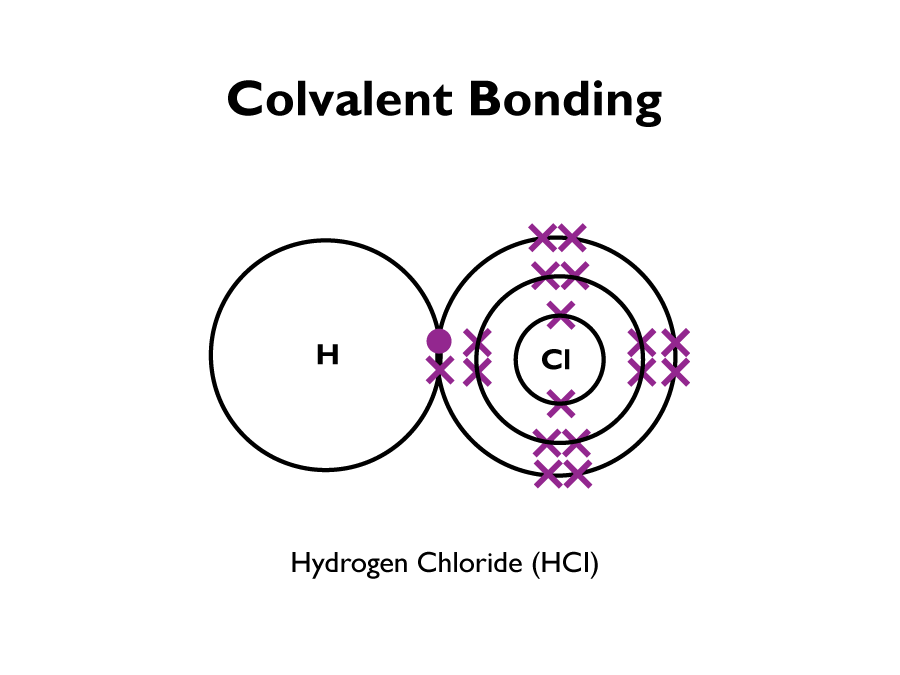
A compound usually has totally different chemical properties than it’s original elements. For example iron sulphite behaves completely different to its constituents iron and sulphur.
Formulas and Equations
Formulas and equations state what atoms are involved in a reaction, and how the substances change during the reaction. Compounds are represented by formulas. They tell us what elements are involved in the reaction, by their symbols, and what proportions they are found in the compound.
Water (H2O), for example, is a compound formed by two atoms of hydrogen, bonding with a single atom of oxygen.
Sometimes formulas have brackets in them. For example Calcium hydroxide, Ca(OH)2. This means that, the number outside the brackets, involves everything inside the brackets. So, there is one atom of calcium, two atoms of oxygen, and two atoms of hydrogen.
An easy way to approach a chemical reaction is to first write a word equation. This then makes it easier to write the symbols of the reaction.
For example:
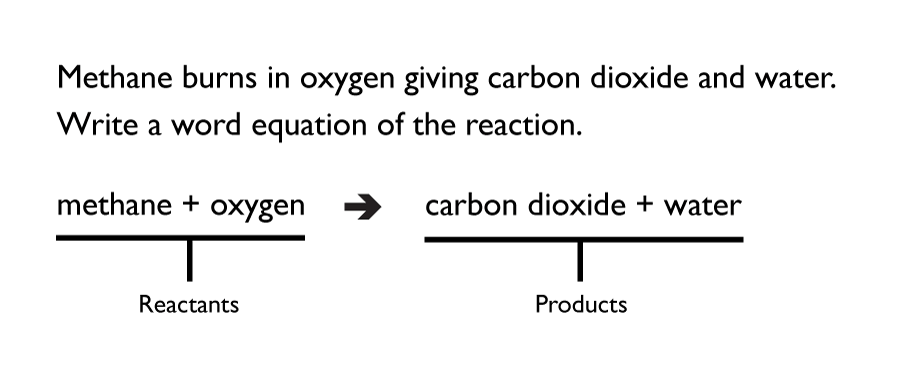
Symbol equations give information on the quantities of the reactants and products. They must be balanced though, by having the same number of atoms on the side of the reactants, as there is on the products.
The symbol equation of reacting sulphuric acid with sodium hydroxide to get sodium sulfate, and water, in its unbalanced state, looks like the following:
H2SO4 + NaOH → Na2SO4 + H2O
Note that there are three atoms of hydrogen, on the side of the reactants, and only two atoms of hydrogen, on the side of the products. They should both be the same amount. Same thing applies to the sodium atom.
So to balance the equation, you must put numbers in front of the atoms, until you get a balanced amount. Therefore:
H2SO4 + 2NaOH → Na2SO4 + 2H2O
… We can put a two in front of the sodium hydroxide, and a two in front of the water molecule, to get a balanced equation. Where the Right-Hand Side (RHS) and the Left-Hand Side (LHS) are both balanced.
Mixtures
Mixtures consists of two or more elements or compounds not chemically combined together. The chemical properties of each substance in the mixture are unchanged.
Mixtures can be separated by physical processes such as filtration, crystallisation, simple distillation, fractional distillation and chromatography. These physical processes do not involve chemical reactions and no new substances are made.
Unlike a compound, there is no chemical bond between the different compounds or elements in the mixture. Compounds are also a lot harder to separate than mixtures.
Air, is a mixture of gases, that can be easily separated. Crude oil is a mixture of different length hydrocarbons that can be separated by fractional distillation.
The properties of a mixture are just like the properties of the separate components.
Chromatography
Paper chromatography is a laboratory technique for separating compounds out of a mixture. It can be used to separate the different dyes out of a solution of ink.
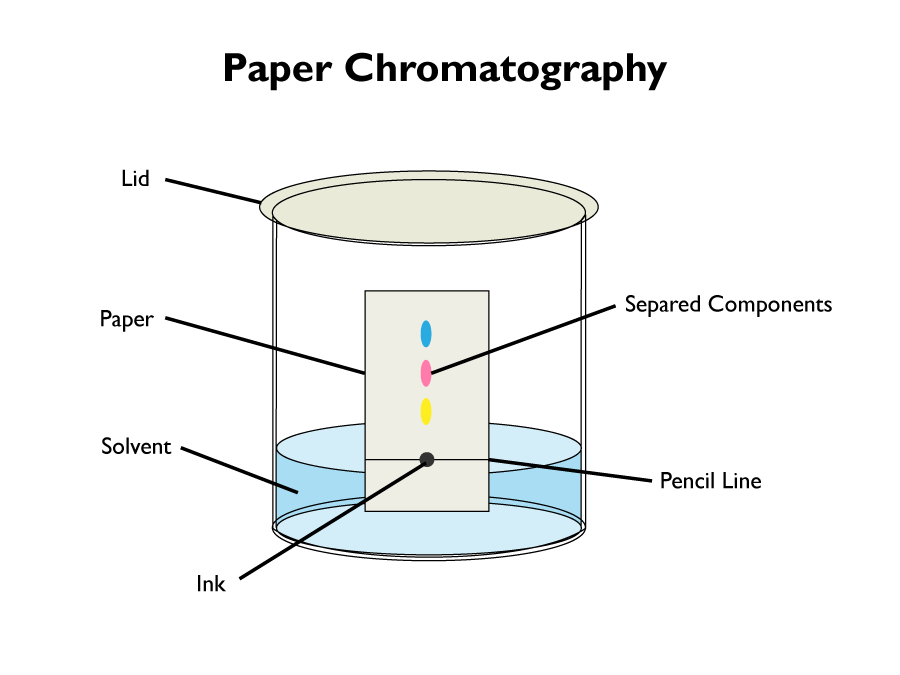
The following steps are performed to extract the dyes from the solution of ink:
- A line is drawn with a pencil, at the bottom of the piece of paper. It is essential to use a pencil, since it wont separate with the ink.
- Add a spot of ink to the middle of the pencil line.
- Place the paper in the solvent. Water should suffice, but sometimes other solvents are needed, for example ethanol.
- Make sure the ink is not touching the solvent. Otherwise it will dissolve into it.
- Place the lid on the container. This stops the solvent from evaporating.
- The solvent should seep up the paper, taking the ink with it.
- The different colour dyes will move up the paper at different rates, separating into different coloured dots as they travel.
- Any dyes that are insoluble, will stay at the baseline.
- When the dyes reach the top of the paper, take it out of the solvent. The result is called a chromatogram.
Filtration and Crystallisation
There are three commonly used ways to separate mixtures in a laboratory. Filtration, evaporation and crystallisation.
Filtration
Filtration is used to separate insoluble compounds from a mixture.
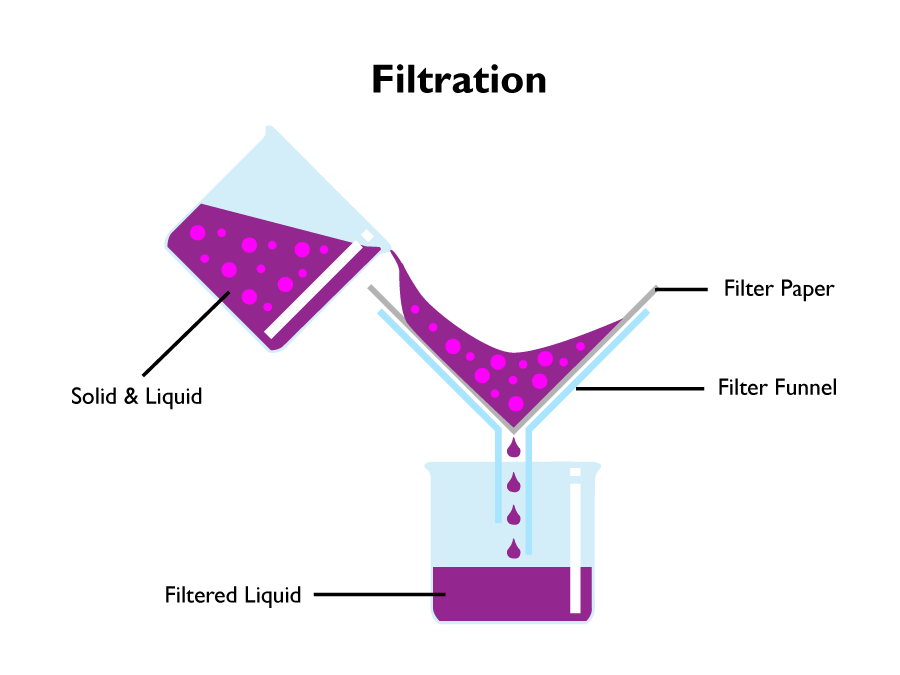
To be able to filter a mixture, there must be insoluble solids in it. Insoluble means it cannot be dissolved into a solution. If the solution contains soluble solids, then either evaporation or crystallisation must be used.
Evaporation
Evaporation is used when you have a soluble solid that has dissolved completely into a solution, and needs to be separated. Soluble, means it can be dissolved.
The steps involved in evaporation are really simple.
- Mix the soluble solid with a solvent until it has dissolved
- Pour the solution onto an evaporating dish.
- Heat the solution with a Bunsen burner.
- As the solution heats up, and evaporates, it will start to become more concentrated.
- Eventually when all the solvent has been evaporated, you should be left with the crystallised solid.
Evaporation is a very effective way of separating a soluble solid from a solvent. However, it is only useful if your solid doesn’t breakdown when heated. If it does, then crystallisation is your best option.
Crystallisation
The following steps should be made when crystallising a soluble solid from a solvent, such as water.
- Pour the solution onto an evaporating dish.
- Heat up the solution with a Bunsen burner, until crystals start to form.
- At the point of crystallisation, remove the dish from the heat and leave the solution to cool.
- Use filtration, to filter the crystals out of the solution.
- leave in a drying oven, on a low heat, to evaporate the last vestiges of the solvent. Until you are left with nice big crystals.
Filtration and crystallisation can be used to separate rock salt from a mixture of salt and sand.
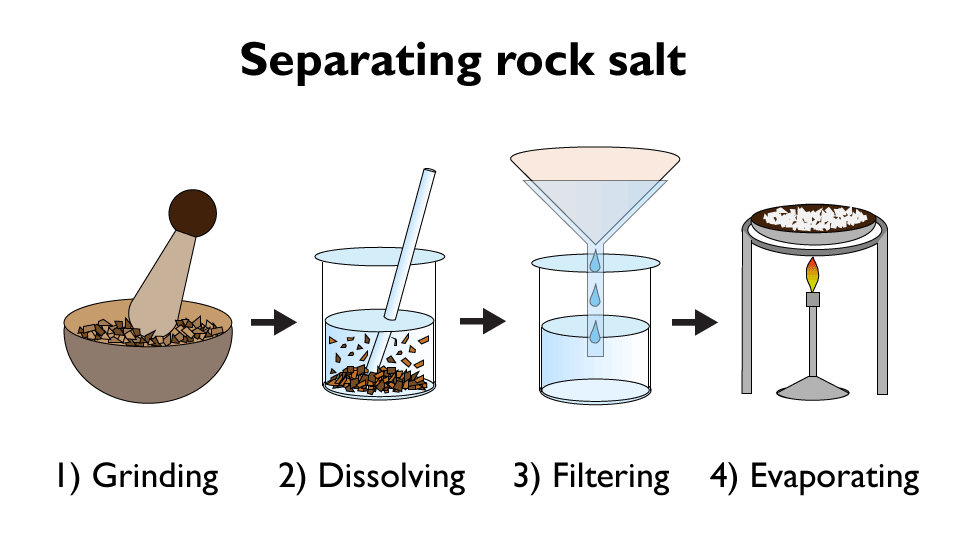
- Firstly, the salt / sand mixture is grind into smaller particles using a pestle and mortar. This will make it dissolve easier.
- The mixture is then put into a solvent, such as water. This will dissolve the salt, but not the sand.
- Then the mixture is filtered. The salt particles are tiny, so they pass through the filter easily. The grains of sand are not so small, so they do not pass through the filter.
- Finally the last stage involves slowly heating the concentrated solution, that is left after filtering. This continues until crystals of salt start to form. If extra large crystals are desired, you can use the crystallisation technique to finish the process off.
Simple Distillation
Simple distillation is used to separate a solvent from a solution. It is effective in removing water, from salt water.
To do this, firstly the solution is heated, then the water evaporates, cools, then condenses. It is then collected. Eventually only salt is left in the flask.
Simple distillation works because the dissolved solute has a higher boiling point then the solvent. When the solution is heated, the liquid with the lowest boiling point evaporates first. The gas vapour is then cooled by the condenser. The liquid that condensers out, is a pure solvent, containing no insoluble solids.
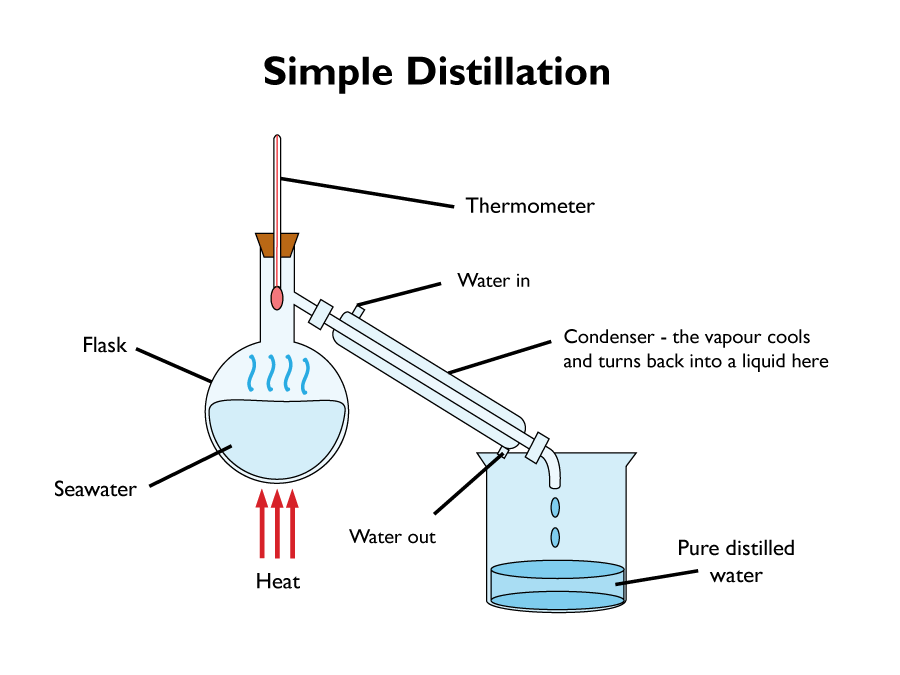
Simple distillation is only really useful for mixtures that have widely different boiling points. If the mixture has liquids of similar boiling points, then fractional distillation should be used instead.
Fractional Distillation
Fractional distillation is useful when separating a mixture of different liquids, that are of boiling points, that are close together. It is particularly useful when separating the different fractions of crude oil.
The diagram bellow shows the fractional distillation of crude oil into various hydrocarbons.
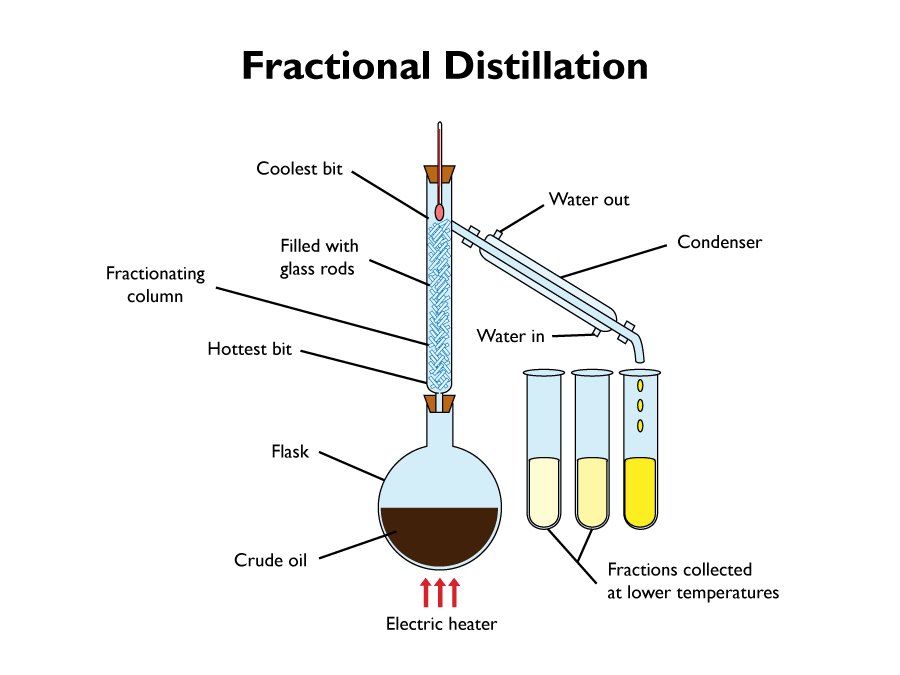
- Pour the crude oil into the flask. Then gently apply heat to it. The different hydrocarbons have different boiling points, so they should evaporate at different temperatures.
- Steadily increase the temperature, until the thermometer reaches the temperature of the liquid, with the lowest boiling point.
- Collect the liquid in a test tube.
- Raise the temperature until it reaches that of the next liquids boiling point.
- Collect the liquid in another test tube.
- Repeat the last two steps until all the liquids have been collected.
It wont matter if liquids with a higher boiling point, start to evaporate at the same time as a liquid with a lower boiling point. This is because, the vapour will only get part of the way up the fractionating column, before cooling, condensing, and running back down into the flask. This is because, the further you go up the fractionating column, the cooler it gets.
Using an electric heater is better than using a Bunsen burner, since it will be easier to control the temperature.
The History of the Atom
Whenever new evidence, in the field of atomic theory has occurred, the structure of the atom has changed.
In the early 19th century John Dalton suggested that atoms were like solid spheres. The spheres making up the different elements.
In 1897 ll Thomson found, from his measurements of charge and mass, that an atom must contain smaller negatively charged particles, called electrons. This changed the solid sphere model.
With the death of the solid sphere model, came the ‘plum pudding model’. With the discovery of the electron, this model posited that an atom was a ball of positive charge, with negatively charged electrons embedded randomly within it.
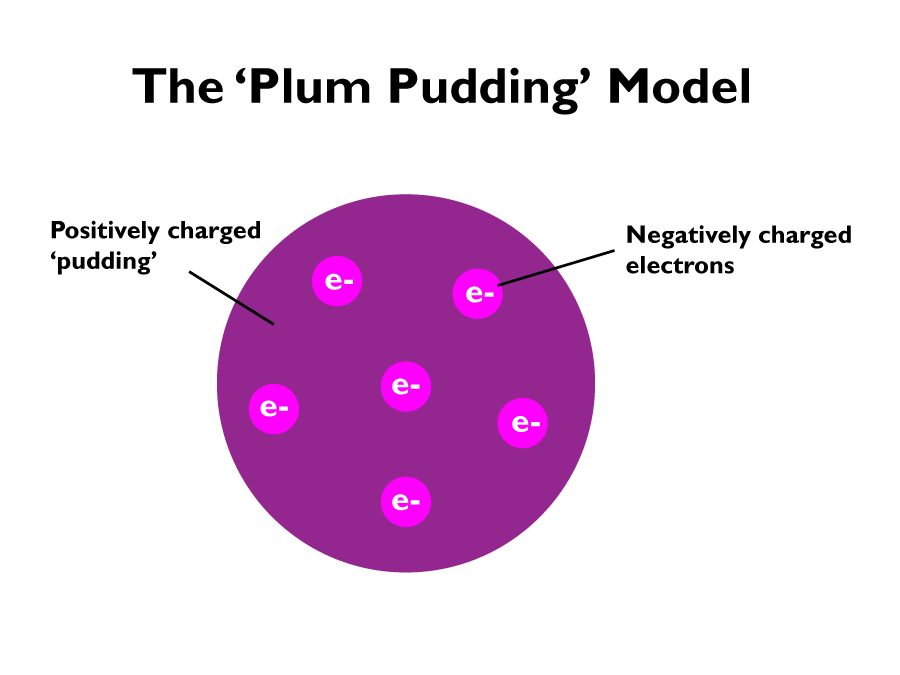
In 1909 Ernest Rutherford and his student Ernest Marsden found that the ‘plum pudding’ model was incorrect.
From their alpha particle scattering experiments; firing positively charged alpha particles at a very thin sheet of gold, led to the conclusion that the mass of an atom was concentrated at the centre (nucleus) and that the nucleus was positively charged. The positively charged alpha particles repelled the positively charged nuclei, since positive to positive charges repel one another. This nuclear model replaced the plum pudding model.
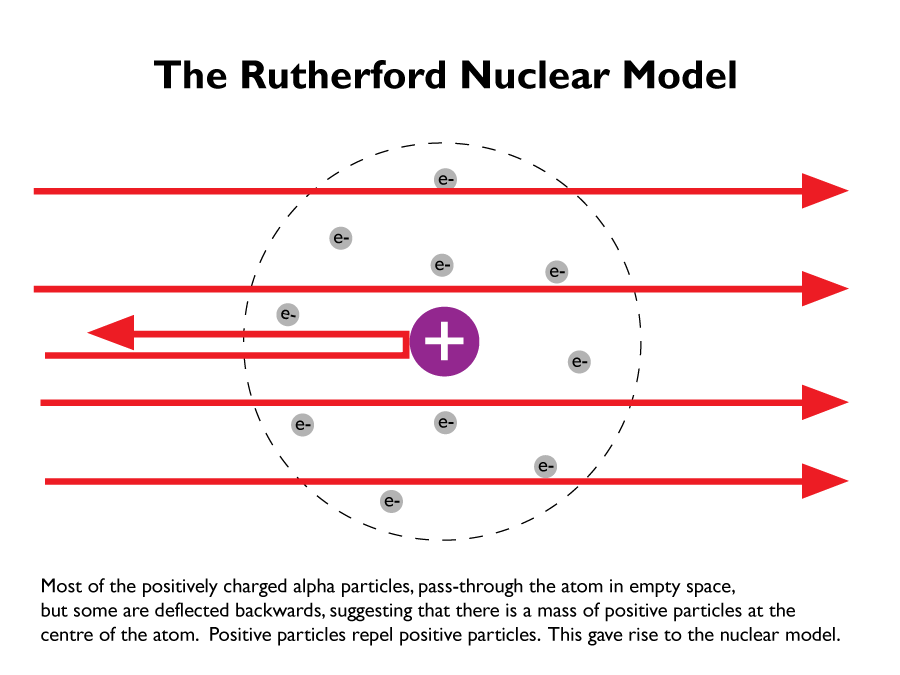
After realising that the nucleus is positive and electrons are negative, scientists assumed that the electrons would be attracted to the nucleus and therefore ‘collapse’.
Niels Bohr suggested that the electrons were in fact in shells, that orbited around the nucleus at fixed distances. Niels theory was supported by many scientific experiments at the time.
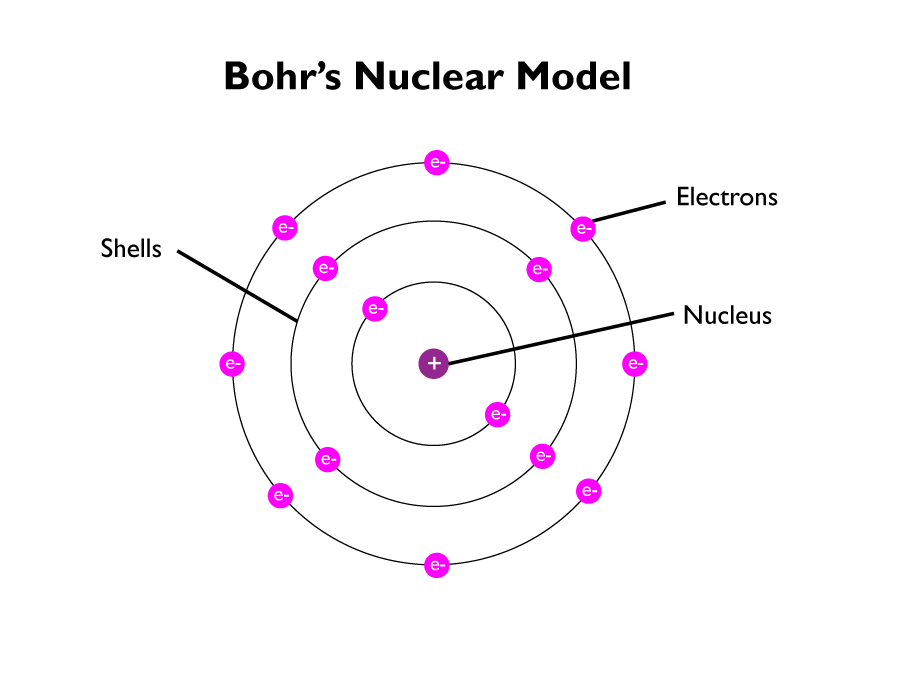
Later experiments led to the idea that the positive charge of any nucleus could be subdivided into a whole number of smaller particles, each particle having the same amount of positive charge. The name proton was given to these particles.
The experimental work of James Chadwick provided the evidence to show the existence of neutrons within the nucleus. This was about 20 years after the nucleus became an accepted scientific idea.
Electronic Structure
The electrons in an atom occupy the lowest available energy levels (innermost available shells). The electronic structure of an atom can be represented by numbers or by a diagram.
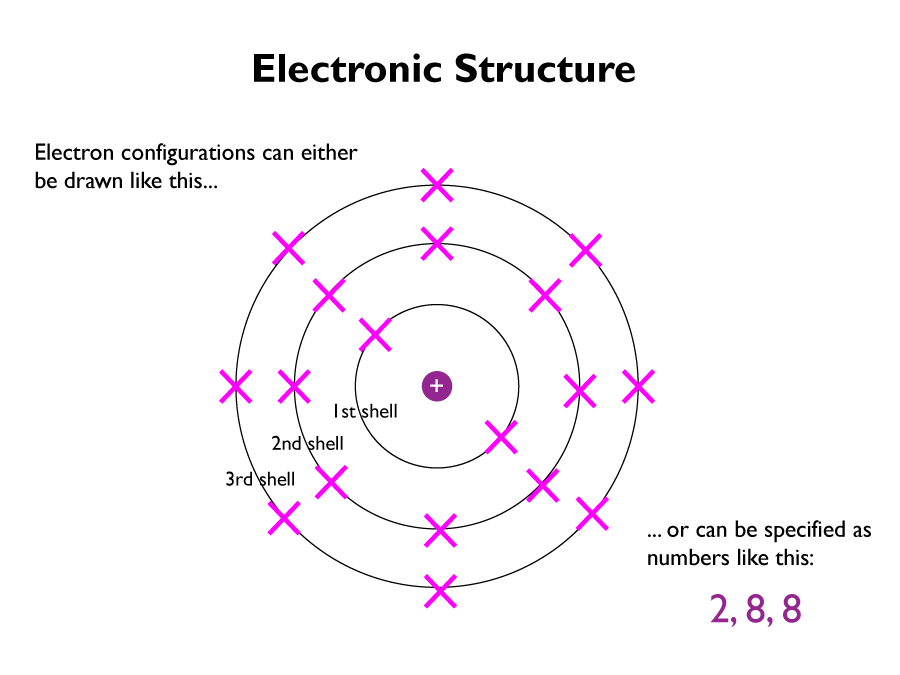
Electrons tend to follow a set of rules (simplified for GCSE level):
- electrons only occupy shells or energy levels
- the lowest levels are filled first
- only a certain number electrons are allowed in certain shells; 2 for the first shell, 8 for the second, and 8 for the third
- Atoms are less reactive when they have full shells of electrons
- In most atoms, having an outer shell that is not full, makes them want to react to fill it
You can then follow these rules to work out the electronic structures that each atom has. For example:
Lithium has an atomic number of 3. This means it has 3 protons, which means it also has 3 electrons. Since there can be only two electrons in the first shell. Then it must be that lithium has an electronic structure of 2, 1. Another example:
Neon has an atomic number of 10. This means it has 10 protons. This also means it has 10 electrons. Since there can only be 2 electrons in the first shell, and 8 in the next shell after that, then the electronic structure must be 2, 8.
Development of the Periodic Table
Before the discovery of protons, neutrons and electrons, scientists attempted to classify the elements into tables by arranging them in order of their atomic weights.
The early periodic tables had elements placed in inappropriate groups, and gaps existed where elements had not been discovered yet.
In 1869, Dmitri Mendeleev left gaps for elements that he thought had not been discovered yet. He also changed the order based on atomic weight.
Some of the gaps allowed Mendeleev to predict what the elements may be. For example, Mendeleev predicted the existence of 'eka-silicon', which would fit into a gap below silicon.
The discovery of isotopes in the early 20th century confirmed Mendeleev’s view to not place elements in a strict order of atomic weight, but also take into account their properties.
Knowledge of isotopes made it possible to explain why the order based on atomic weights was not always correct.
The Modern Periodic Table
The periodic table came to look like it does today, after the discovery of the structure of an atom.
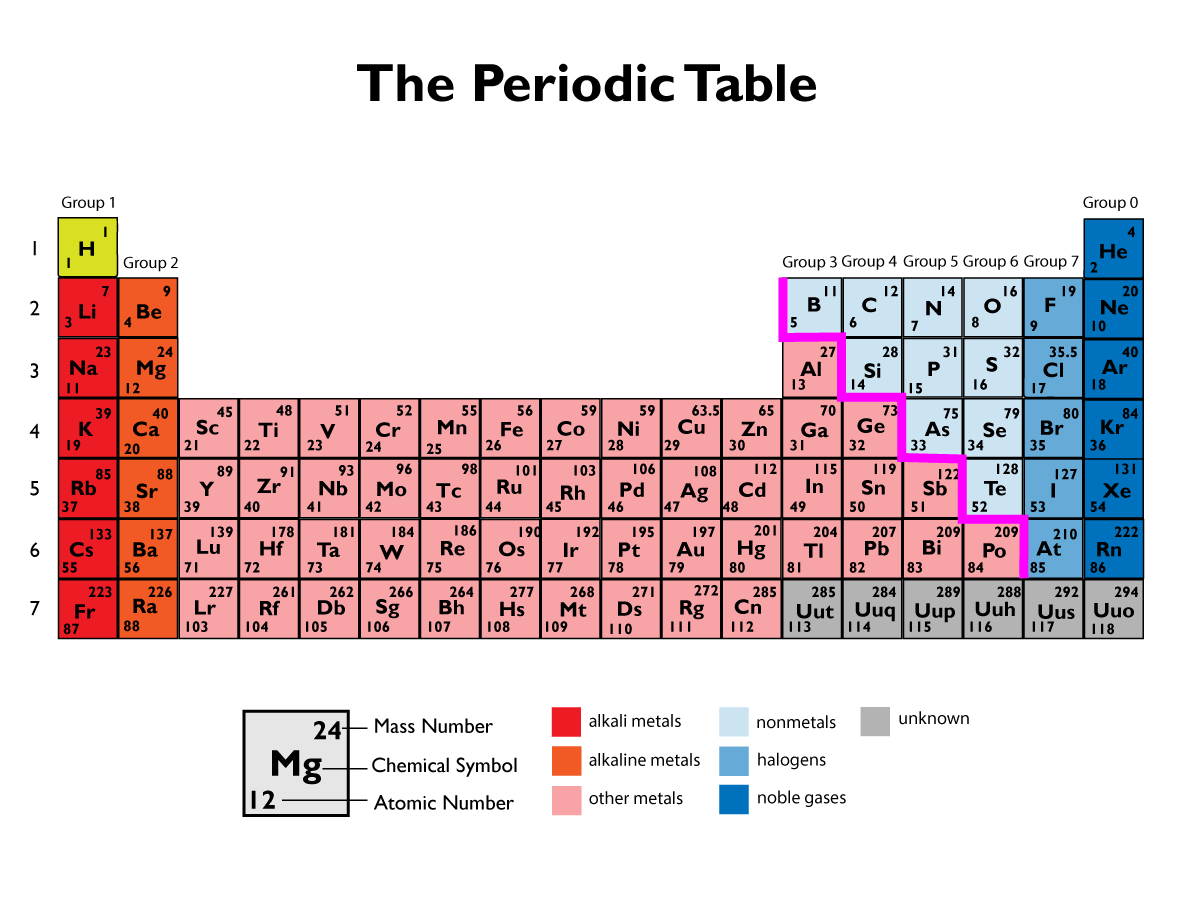
Elements with similar properties are arranged into columns, called groups. The group number tells you how many electrons there is in the outer shells of those elements. The elements in a group all react in a similar way. You can use this information to predict how other elements might react.
Metals tend to be found on the left, non-metals on the right.
The rows of the periodic table are called periods, and are arranged in order of increasing atomic number. Each new period also represents another full shell of electrons.
| Electronic structure feature | Link to the periodic table |
|---|---|
| Number of shells | Period number |
| Number of electrons in outermost shell | Group number |
| Total number of electrons in all shells | Atomic number |
The periodic table got its name from elements properties occurring at regular intervals.
Metals and Non-Metals
Most elements on the periodic table are metals. Metals are found on the left of the periodic table, and non-metals are to the right.
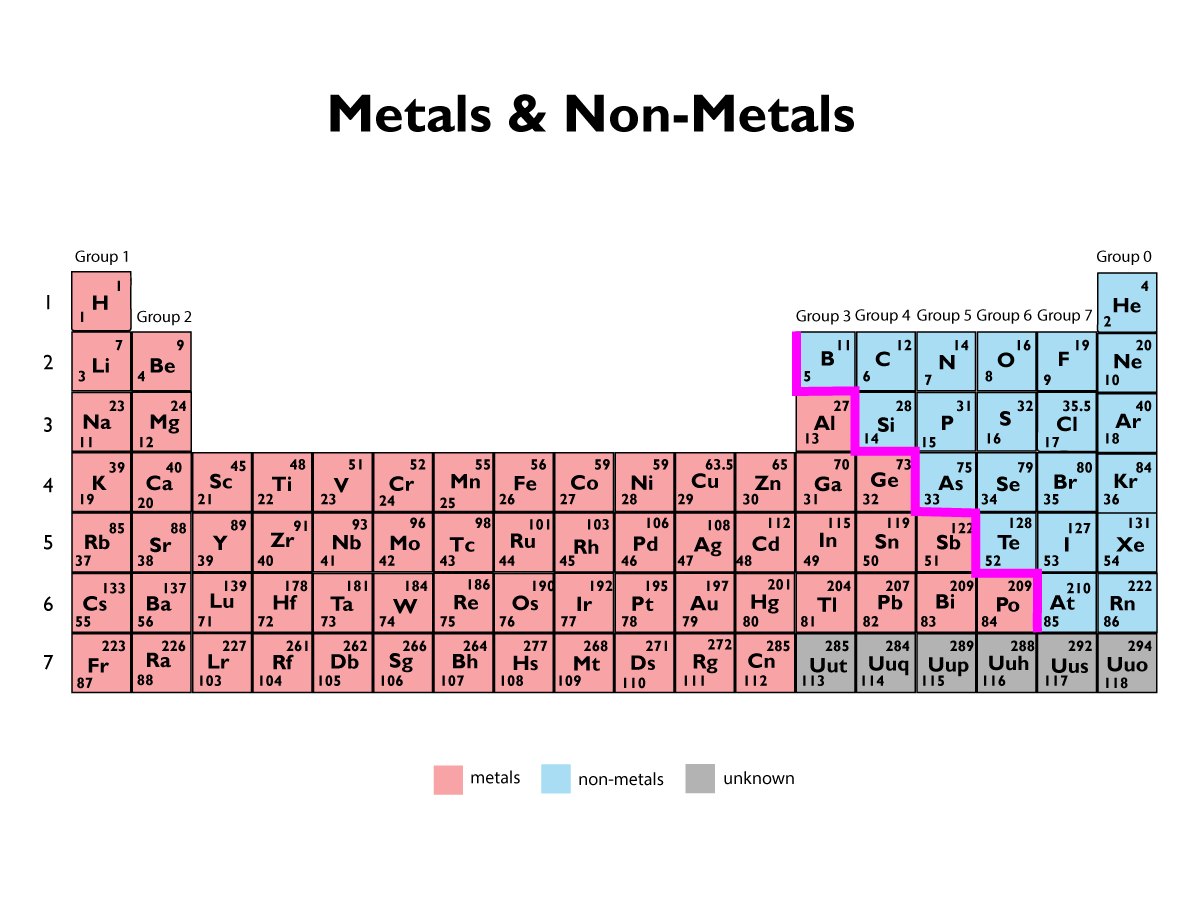
Elements that react to form positive ions are metals. Elements that do not form positive ions are non-metals.
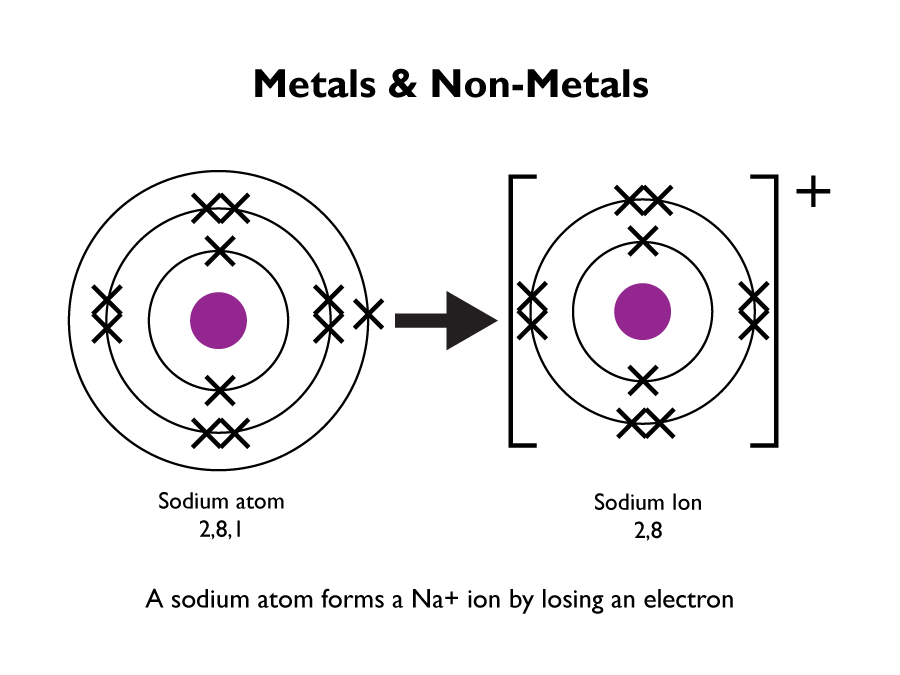
Metal and non-metal elements have different physical properties:
- most metals have high melting and boiling points
- most non-metals have low melting and boiling points
- most metal oxides are basic
- most non-metal oxides are acidic
- metals are great at conducting heat and electricity
- non-metals don’t conduct heat or electricity well
| Properties of a typical solid metal | Properties of a typical solid non-metal |
|---|---|
| Good conductor of electricity | Poor conductor of electricity |
| Good conductor of heat | Poor conductor of heat |
| Shiny | Dull |
| High density | Low density |
| Malleable | Brittle |
| Ductile | Brittle |
When atoms react they lose, gain or share electrons whilst trying to form full outer shells. Metals to the left of the periodic table, don’t have many electrons to remove, they therefore react easier. Metals to the bottom of the periodic table have outer electrons that are a long distance to the nucleus, therefore it doesn’t take much energy to remove those electrons in a reaction. Making it easier to form positive ions, with a full outer shell.
For non-metals forming positive ions is much harder. This is because their outer electrons are much closer to the nucleus, they therefore have a stronger bond. They also have lots of electrons to remove to get to the outer shell making them less reactive.
Group 0 Elements
Group 0 elements are called the noble gases. They are very inert, which means they don’t react with anything that well.
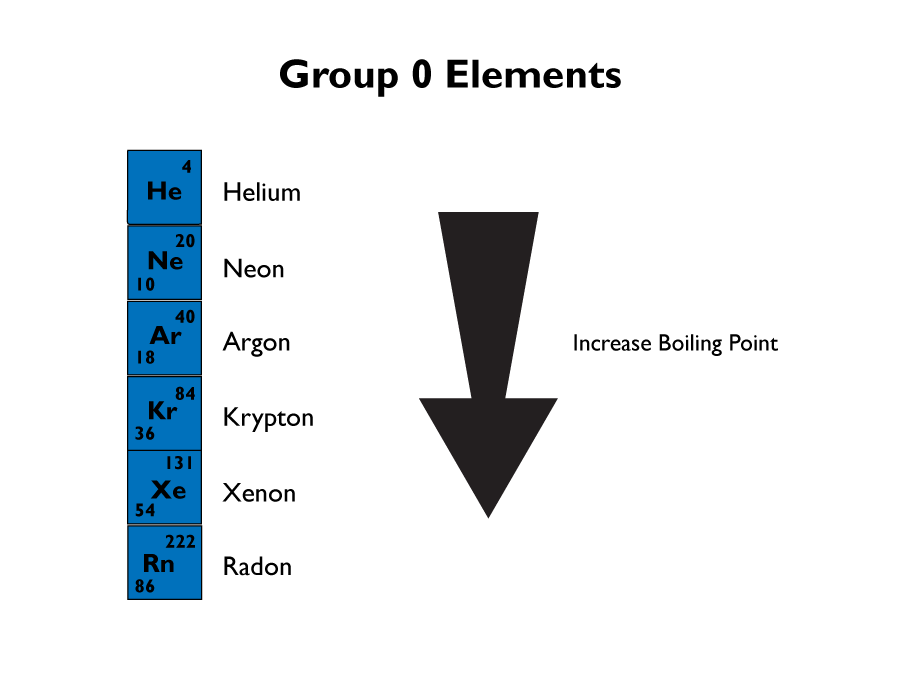
The reason they are inert is because they mostly have a full outer shell of electrons, which means they don’t need to gain, loose or share electrons to fill their outer shells. This makes them very stable.
All of the groups gases are colourless at room temperature. They are also non-flammable, this is because they are inert.
The boiling point of the noble gases increases as you move down the group. The atomic mass also increases. There is also an increase in the amount of electrons in each atom, leading to an increase in the intermolecular forces which need to be overcome. This is why the boiling point increases as you go down the group.
Group 1 Elements
Group 1 elements are known as the Alkali metals. They are very reactive which is why they need to be kept in oil, and handled with forceps. They are all soft to touch, and have a low density.
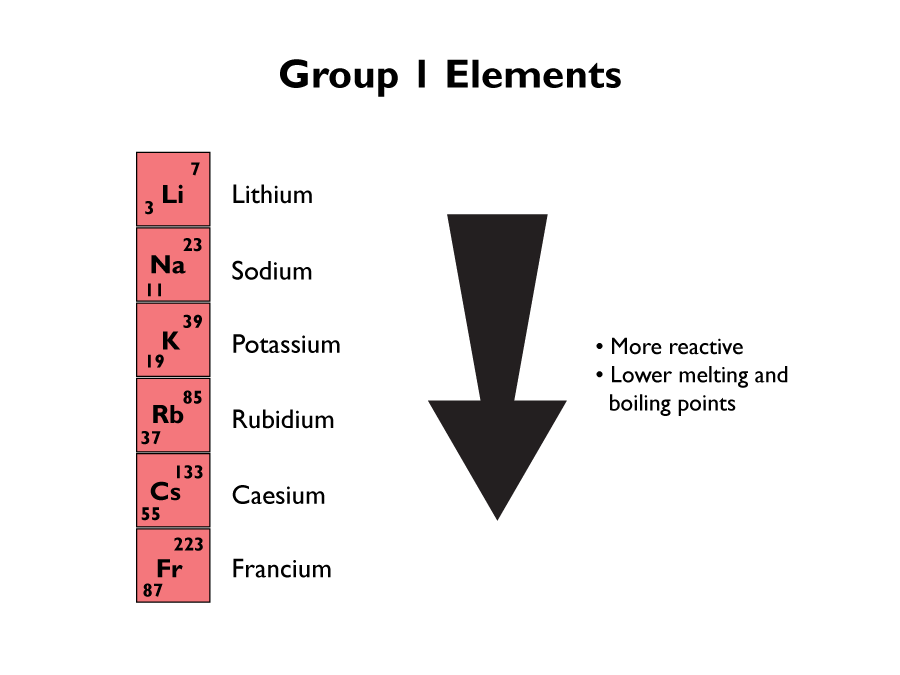
They have a single electron in their outer shell, which accounts for there reactivity. They also have more shells of electrons, as you go down the group, which means they have less attraction to the nucleus, so are more easily given away in a reaction.
Reactions with non-metals produces an ionic compound. They readily form 1+ ions.
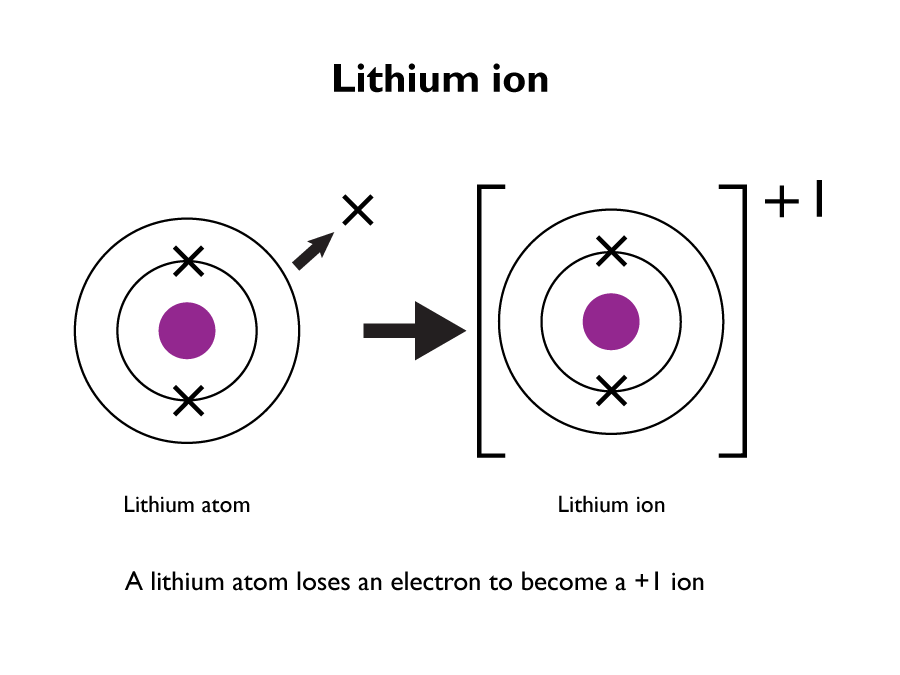
Reaction of sodium with water:
2Na(s) + 2H2O(I) → 2NaOH(aq) + H2(g)
sodium + water → sodium hydroxide + hydrogen
All group 1 metals react violently in water. The reaction produces an alkaline solution and hydrogen gas. The further down the periodic table an element is, the more violent the reaction, and the amount of energy given off is greater.
Reaction of sodium with chlorine:
2Na(s) + Cl2(g) → 2NaCl(s)
sodium + chlorine → sodium chloride
When heated in chlorine gas, group 1 metals also react violently, producing sodium chloride (salt).
Group 7 Elements
Group 7 elements are called the halogens. They are non-metal and have colourful vapours.
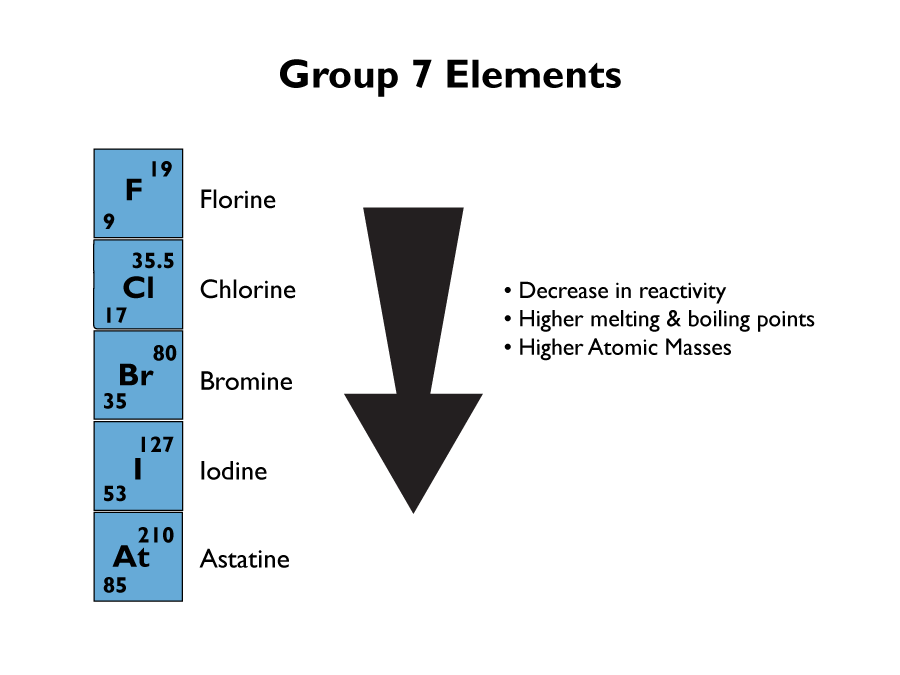
Group7 elements become less reactive the further down the group. This is because the outer shell is further from the nucleus, so it is harder to gain an extra electron. Also the melting and boiling points go up the further down group 7 you go. All the elements in group 7 have seven electrons in their outer shells, so they all react in similar ways.
More reactive halogens will displace lesser reactive halogens in a reaction. A displacement reaction can happen between a more reactive halogen and the salt of a less reactive one.
Take for instance the following reactions. Chlorine will displace bromine and iodine from an aqueous solution of its salt (bromide or iodide).
Cl2(g) + 2KI(aq) → I2(aq) + 2KCl(aq)
Cl2(g) + 2KBr(aq) → Br2(aq) + 2KCl(aq)